In the world of medical technology, innovation is only half the story. A device may be technically brilliant, but if users struggle to operate it safely, the consequences can be serious. That’s why human factors and usability engineering are essential components of medical device development. One of the most powerful tools in that toolkit is SHERPA.
What is SHERPA?
SHERPA stands for Systematic Human Error Reduction and Prediction Approach. Originally developed in high-risk industries such as nuclear power and aviation, SHERPA offers a structured way to anticipate how users might make errors and how to design the causes of those errors out of the system.
At its core, SHERPA is built on task analysis. This begins by breaking a procedure or user interaction down into its component steps. Then, each step is assessed for potential error modes: what could go wrong, why it might happen, and what the consequences would be. The SHERPA framework uses an error taxonomy such as action errors, checking errors, selection errors, and information communication errors, which ensures that every type of failure is considered.
Why Use SHERPA in Medical Device Design?
Medical devices are used in complex, high-stakes environments, often by clinicians under time pressure, so predicting and mitigating user error is vital. SHERPA provides design teams with a methodical approach to identifying risks in user–device interactions, long before the product reaches the market.
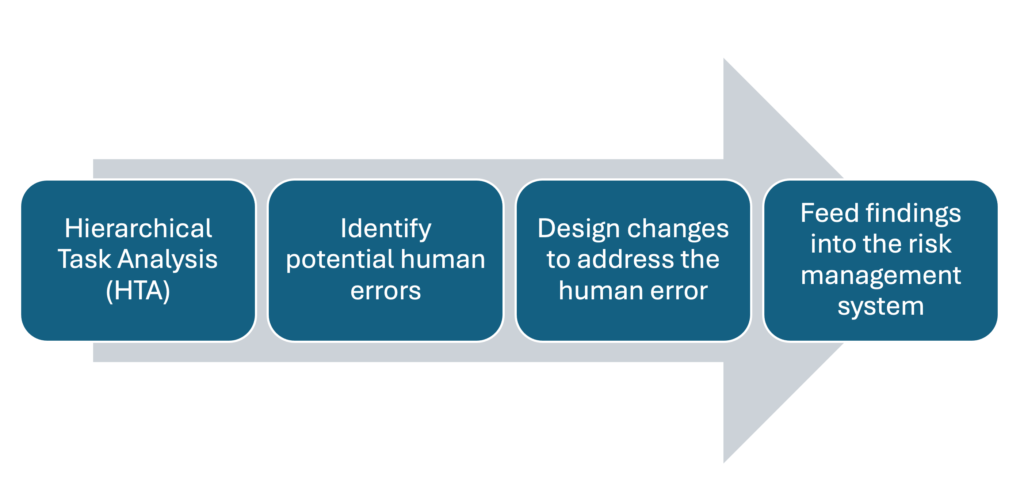
Here’s how SHERPA fits into the development process:
- During the early design stage, teams carry out a Hierarchical Task Analysis (HTA) to map how users set up, operate, and maintain the device.
- Each task step is then examined using SHERPA to identify potential human errors.
- For each identified risk, the team proposes design changes or controls, such as simplifying the interface, adding an alert, or reordering the sequence of steps.
- The outcomes of this analysis directly inform the usability engineering file and feed into the risk management system.
Real-World Applications
SHERPA isn’t just a theoretical tool, it’s about making devices safer:
- In one study, SHERPA was used to analyse chemotherapy administration processes in an oncology ward. Analysts identified 109 potential errors, most related to task execution, and helped implement changes that improved patient safety.1
- In a separate investigation, SHERPA helped uncover why a near-miss involving incorrect flush fluid in an arterial line setup occurred. The analysis revealed not only the breakdown in task execution but also the contextual pressures that made the error more likely.2
- Several device manufacturers have applied SHERPA to meet regulatory requirements during development, using it to systematically identify and manage use-related hazards.3
Benefits and Limitations of SHERPA in Medical Device Design
Like any method, SHERPA comes with both strengths and challenges.
The benefits are clear: SHERPA helps teams uncover hidden risks in user–device interactions early in the design process. This proactive approach supports compliance with standards like ISO 14971 Medical Devices – Application for Risk Management to Medical Devices and IEC 62366-1 Application of Usability Engineering to Medical Devices, both of which emphasise identifying and mitigating use-related risks. Because SHERPA focuses on task-level analysis, it also guides design decisions, leading to more intuitive, safer products.
However, because it is a predictive (rather than an observational method), it must always be used with empirical usability testing tools such as formative and summative analyses
When integrated into a broader human factors programme, SHERPA is a powerful tool that helps bridge the gap between user needs and safe design. It provides the detail and structure that many traditional risk analyses lack, making it especially valuable in the context of safety-critical devices.
Meeting the Standards
SHERPA directly supports key requirements in IEC 62366 (usability engineering) and ISO 14971 (risk management), particularly in identifying and managing use-related risks. Its structured task-based analysis complements traditional hazard and failure mode assessments, offering a clear, traceable method for documenting how potential use errors were considered and addressed.
This approach helps build a robust usability engineering file and risk management report, providing the kind of evidence regulators increasingly expect to see. By integrating SHERPA into the development process, teams can demonstrate that human factors have been addressed systematically and proactively.
Conclusion
SHERPA is a structured, evidence-based method that strengthens human factors practice in medical device development. By proactively identifying how users might go wrong and designing to prevent those errors, SHERPA helps create devices that are not only innovative, but genuinely safe and usable.
In a sector where even a minor error can have life-altering consequences, SHERPA gives design teams the insight they need to make every user interaction safer.
References
- Teymourzadeh, E., et al. (2023). Assessment and Reduction of Human Error using SHERPA Technique in Chemotherapy Department of a Large Military Hospital. International Journal of Occupational Hygiene, 15(3), 1–10.
- Healthcare Safety Investigation Branch (HSIB). (2021). The use of an appropriate flush fluid with arterial lines.
- Embrey, D. (2014). Application of SHERPA to Predict and Prevent Use Error in Medical Device Design. Proceedings of the Human Factors and Ergonomics Society Annual Meeting, 58(1), 1047–1051.
Looking for Human Factors support in medical device design?
Discover how we can help you navigate the process with confidence.
Want to see SHERPA in action?
Book a demo to explore how it supports safety, quality, and performance in complex environments.